
Full resolution (JPEG) - On this page / på denna sida - Sidor ...
<< prev. page << föreg. sida << >> nästa sida >> next page >>
Below is the raw OCR text
from the above scanned image.
Do you see an error? Proofread the page now!
Här nedan syns maskintolkade texten från faksimilbilden ovan.
Ser du något fel? Korrekturläs sidan nu!
This page has never been proofread. / Denna sida har aldrig korrekturlästs.
Teknisk Tidskrift
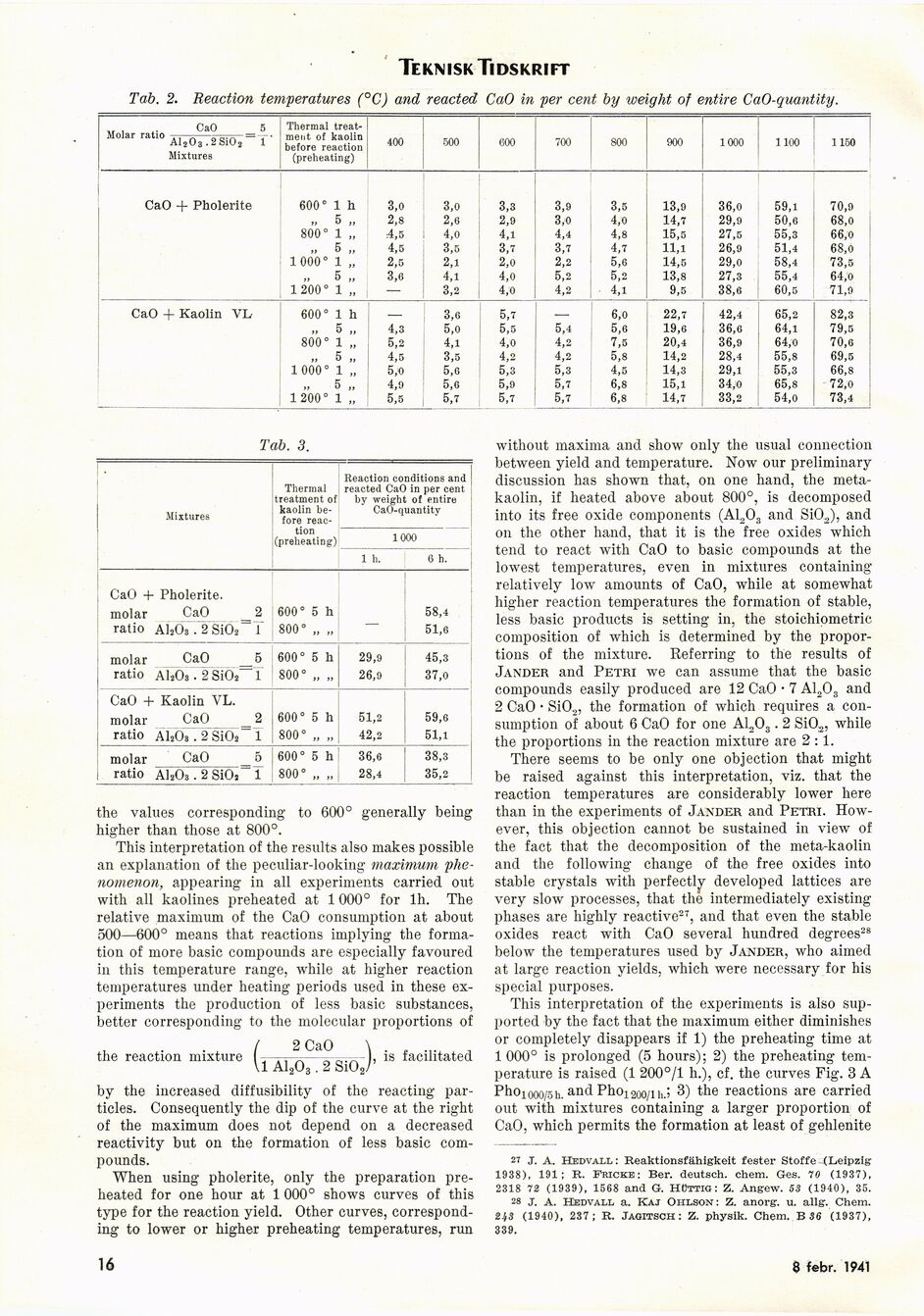
Tab. 2. Reaction temperatures (°C) and reacted CaO in per cent by weight of entire CaO-qiiantity.
Ca0 5 Molar ratio —.... =-• AI2O3.2S1O2 1 Mixtures Thermal
treat-ment of kaolin before reaction (preheating) 400 500 600 700 800 900 1000 1100 1150
CaO + Pholerite 600° 1 h 3,0 3,0 3,3 3,9 3,5 13,9 36,0 59,1 70,9
ii 5 2,8 2,6 2,9 3,0 4,0 14,7 29,9 50,6 68,0
800° 1 4,5 4,0 4,1 4,4 4,8 15,5 27,5 55,3 66,0
„ 5 4,5 3,5 3,7 3,7 4,7 11,1 26,9 51,4 68,0
1 000° 1 »» 2,5 2,1 2,0 2,2 5,6 14,5 29,0 58,4 73,5
5 i> 3,6 4,1 4,0 5,2 5,2 13,8 27,3 55,4 64,0
1200° 1 „ — 3,2 4,0 4,2 4,1 9,5 38,6 60,5 71,9
CaO + Kaolin YL 600° 1 h ___ 3,6 5,7 — 6,0 22,7 42,4 65,2 82,3
„ 5 ii 4,3 5,0 5,5 5,4 5,6 19,6 36,6 64,1 79,5
800° 1 5,2 4,1 4,0 4,2 7,5 20,4 36,9 64,0 70,6
5 4,5 3,5 4,2 4,2 5,8 14,2 28,4 55,8 69,5
1 000° 1 H 5,0 5,0 5,3 5,3 4,5 14,3 29,1 55,3 66,8
„ 5 4,9 5,6 5,9 5,7 6,8 15,1 34,0 65,8 72,0
1 200° 1 » 5,5 5,7 5,7 5,7 6,8 14,7 33,2 54,0 73,4
Tab. 3.
Mixtures Thermal treatment of kaolin
before reaction (preheating) Reaction conditions and reacted CaO in per cent by weight of entire CaO-quantity
1000
1 h. 6 h.
CaO + Pholerite.
molar CaO 2 600° 5 h 58,4
ratio AlsOs . 2 Si02 1 800° „ „ 51,6
molar CaO 5 600° 5 h 29,9 45,3
ratio AI2O3. 2 Si02 1 800° „ „ 26,9 37,0
CaO + Kaolin VL. molar CaO 2 600° 5 h 51,2 59,6
ratio AI2O3. 2 SiOa 1 800° „ „ 42,2 51,1
molar CaO 0 600° 5 h 36,6 38,3
ratio AI2O3. 2 SiOa 1 800° „ „ 28,4 35,2
the values corresponding to 600° generally being
higher than those at 800°.
This interpretation of the results also makes possible
an explanation of the peculiar-looking maximum
phe-nomenon, appearing in all experiments carried out
with all kaolines preheated at 1000° for lh. The
relative maximum of the CaO eonsumption at about
500—600° means that reactions implying the
formation of möre basic compounds are especially favoured
in this temperature range, while at higher reaction
temperatures under heating periods used in these
experiments the production of less basic substances,
better corresponding to the molecular proportions of
(2 CaO \
—o o-n )’ facilitated
1 AI2O3 . 2 öi02/
by the increased diffusibility of the reacting
par-ticles. Consequently the dip of the curve at the right
of the maximum does not depend ön a decreased
reactivity but 011 the formation of less basic
compounds.
When using pholerite, only the preparation
preheated for one hour at 1 000° shows curves of this
type for the reaction yield. Other curves,
corresponding to lower or higher preheating temperatures, run
without maxima and show only the usual connection
between yield and temperature. Now our preliminary
discussion has shown that, ön one hand, the
meta-kaolin, if heated above about 800°, is decomposed
into its free oxide components (A1203 and Si0,2), and
011 the other hand, that it is the free oxides which
tend to react with CaO to basic compounds at the
lowest temperatures, even in mixtures containing
relatively löw amounts of CaO, while at somewhat
higher reaction temperatures the formation of stable,
less basic products is setting in, the stoichiometric
composition of which is determined by the
proportions of the mixture. Referring to the results of
Jander and Petri we can assume that the basic
compounds easily produced are 12 CaO • 7 A1203 and
2 CaO • Si0.2, the formation of which requires a
eonsumption of about 6 CaO for one AI203. 2 Si02, while
the proportions in the reaction mixture are 2:1.
There seems to be only one objection that might
be raised against this interpretation, viz. that the
reaction temf>eratures are considerably lower here
than in the experiments of Jander and Petri.
However, this objection cannot be sustained in view of
the fact that the decomposition of the meta-kaolin
and the following change of the free oxides into
stable crystals with perfectly developed lattices are
very slow processes, that the intermediately existing
phases are highly reactive27, and that even the stable
oxides react with CaO several hundred degrees28
below the temperatures used by Jander, who aimed
at large reaction yields, which were necessary for his
special purposes.
This interpretation of the experiments is also
sup-ported by the fact that the maximum either diminishes
or completely disappears if 1) the preheating time at
1000° is prolonged (5 hours); 2) the preheating
temperature is raised (1 200°/l h.), cf. the curves Fig. 3 A
Phoi 000/5 h. and Phoi 200/1 h.j 3) the reactions are carried
out with mixtures containing a larger proportion of
CaO, which permits the formation at least of gehlenite
27 J. A. Hedvall: Reaktionsfähigkeit fester Stoffe (Leipzig
1938), 191; R. Fricke : Ber. deutseh. chem. Ges. 10 (1937),
2318 72 (1939), 1568 and G. Hüttig : Z. Angew. 53 (1940), 35.
28 J. A. Hedvall a. Kaj Ohlson: Z. anorg. u. allg. Chem.
2iS (1940), 237; R. Jagitsch: Z. physik. Chem. B 86 (1937),
339.
16
12 april 1941
<< prev. page << föreg. sida << >> nästa sida >> next page >>